This project was carried out with the invaluable collaboration of

please see a full listing of all contributors at the bottom of this post

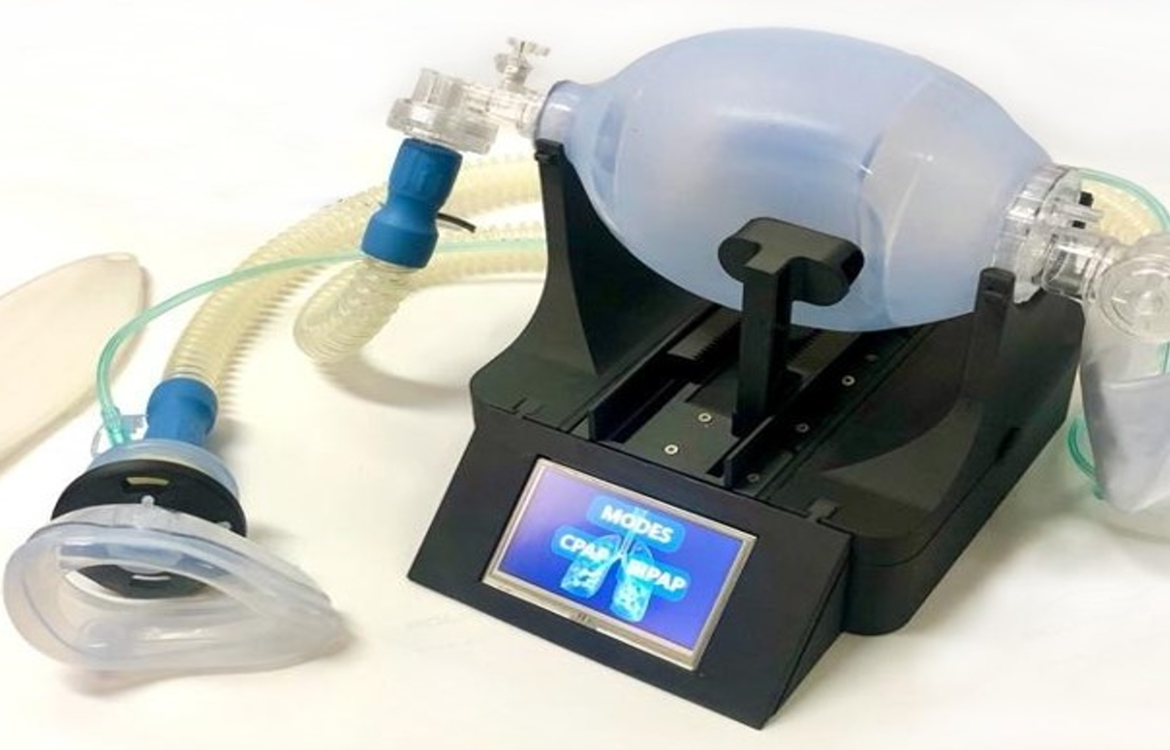
the completed BiPAP Ventilator ready for use
The Need
An early challenge for first-responders of the COVID-19 pandemic was the availability of both Continuous Positive Airway Pressure (CPAP) and Bilevel Positive Airway Pressure (Bi-PAP) portable & easy-to-implement ventilators en-route to ICU. In response to the shortage of ventilators in the early stages of the coronavirus pandemic, the need for the design of a low-cost, portable ventilator that automatically inflates and deflates an Ambu-Bag, as per individual requirements, was initiated by AEDG Students under the AMTC unit, backed up with Mer SETA funding support.
A report in March 2020 by News24 argued that South Africa (SA) appeared to be woefully underprepared for any serious escalation of Covid-19 cases, with roughly 3,000 out of about 7,000 critical care beds available between the public and private healthcare sectors, according to best estimates. Worryingly, the SA Managing Director (MD) of Draeger, a German producer of ventilators worldwide, stated that there were currently no manufacturers of ventilators in South Africa, which relied completely on suppliers who import the machines from various countries including the US, Germany, and China.
The grim reality is that there was a global undersupply of ventilators and Africa, and by extension South Africa, was not on the top of supply lists. There were an estimated 4,000 ventilators in the private health sector, and about half that number in the public sector.
Renai Moothilal, executive director of the National Association of Automotive Component and Allied Manufacturers (NAACAM), who were also busy with a ventilator project, stated that not all the ventilators in SA would be available to Covid-19 patients exclusively since many were already in other critical use. Even if ventilators had become available from international sources, it would've been difficult to get these ventilators to SA since all international flights were suspended and no goods were allowed in and out of the country. Hence the opportunity to urgently develop local, portable, and low-cost automated ventilators existed.
Project Objective
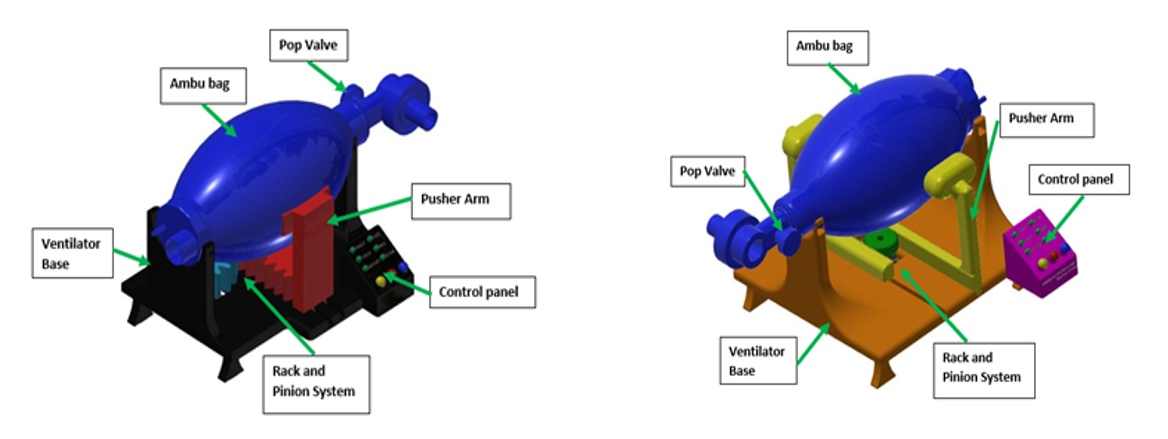
The ventilator is derived from the open-source MIT Bag Valve Mask platform that meets international clinical requirements for a medical respiratory device. Automating the procedure of compression appeared to be the simplest strategy to satisfy the need for low-cost mechanical ventilation, with the ability to also be rapidly manufactured at scale, and deployed quickly for first responders.
Producing this via available in-house Additive Manufacturing (AM) resources would also be substantially cheaper than the conventional ventilator currently imported into the country at significantly elevated cost.
The aim of this project was to provide a mobile, portable functional BiPAP Ventilator that could be utilized by first responders en-route to Hospital ICU's or for in-hospital emerency response.
Early Development
In the beginning of 2021, in-depth research was conducted on ventilators to ensure that the operational procedure an
The articulation system started off with a single pusher arm that inflated and deflated the Ambu-bag as seen above left. The pusher arm has a flat and large surface area to ensure maximum deflation. Initial challenges with this configuration of pusher arm was that, as the arm engaged the bag, the bag would fold into the base of the pusher arm making it harder to compress, therefore the pusher arm had to be redesigned as seen above right.
The next iteration of the pusher arm then consisted of a much finer rack and the arms have a more rounded contact-piece of material that bulges out of the top face of the arm. This eradicated the folding issue faced initially with the Ambu-bag.
From this point onwards a series of shape optimization analysis on the pusher arms were conducted using the Altair Inspire platform until the pusher arm reached the stage as seen below.
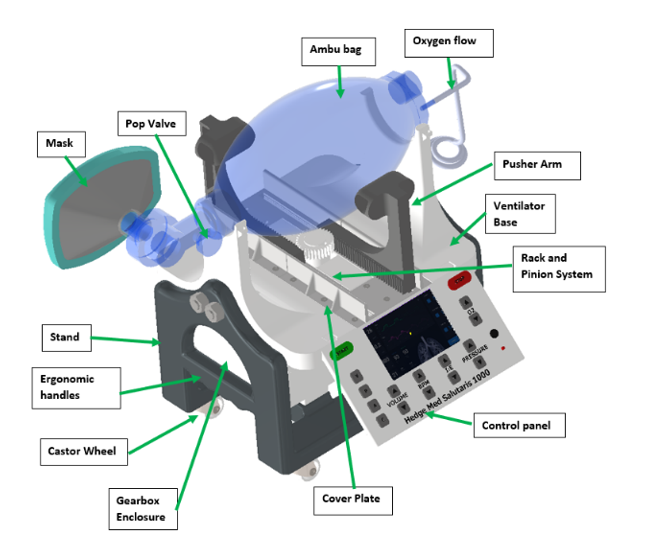
The ventilator in its current form consists of a two-pusher arm rack-and-pinion drive system, which is activated by a servo motor. This is supported by an electronic system consists of an ESP32 microcontroller and an HMI interface that allows the user to alter ranges of settings on the ventilator.
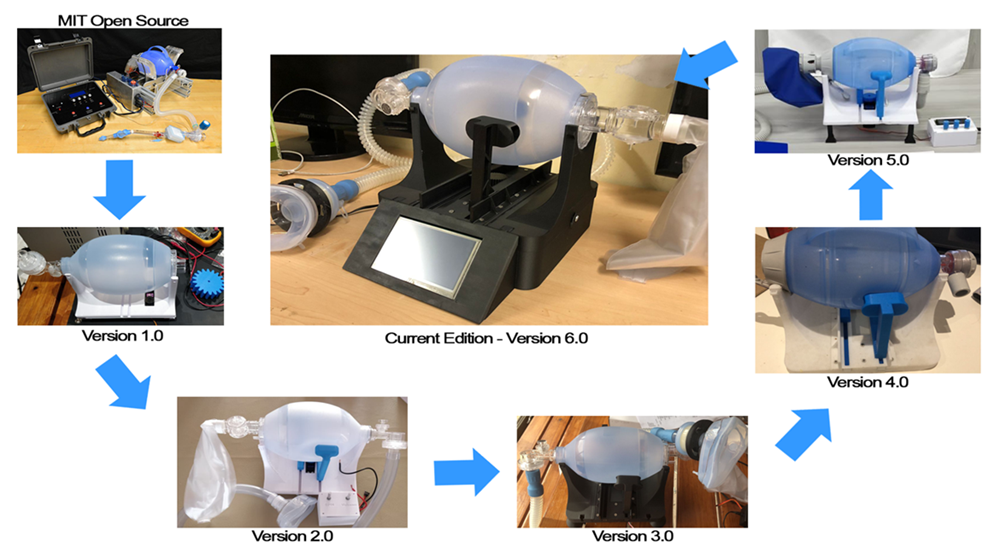
The prototype has undergone multiple iterations as seen below, with each advancement undergoing thorough testing and vetting before committing to the next development step; this is to ensure that all development steps were boilerplate before committing to the next level of development.
The current 6th version, in full BiPAP phase, is now in the process of the next developmental step which is further optimization of the device as part of a Masters study currently in progress with designer & engineering student Zaahid Imran.
Ventilation 101
When a person’s lungs inhale and exhale air normally, oxygen is taken into the cells to survive and carbon dioxide is expelled. A patient suffering from Covid-19 in severe cases has an inflamed airway which essentially causes the lungs to drown in fluids. A mechanical ventilator helps pump oxygen into the lungs at a rate required for normal breathing.
A ventilator is a machine that helps a patient breathe if the patient is unable to do so by themselves. The machine pushes air and oxygen into and out of the lungs. These ventilators are normally referred to as mechanical ventilators or respirators and the main function is to take over the breathing system of a patient using tubes and masks allowing the patient to breathe while they are fighting off infection. A ventilator is essentially a pump delivering air by positive pressure to the patient. For the ventilator to know how much air it must administer into the patient per breath, it must be controlled beforehand by adjusting the required settings. All the modes of control in a full-blown ventilator are time controlled but are limited by volume or pressure. Finally, the ventilator must also be activated by the patient as to when starting delivery of oxygen, and this is what is known as triggering.
Mechanical ventilators may be categorized into the following:
-
Invasive where a tube is inserted into the patient’s airway.
-
Non-invasive where a mask is put over the face covering the mouth and the nose.
Non-invasive ventilators may be further classified into CPAP, BI-PAP and Bag ventilators.
-
CPAP machines are continuous positive Airway pressure - the devices continuously (nonstop) push oxygenated air non-invasively, meaning the device pushes air in during both inhalation and exhalation.
-
BI-PAP machines are CPAP machines but have two different pressures. Inhalation has a higher pressure and exhalation has lower pressure but still continuously supplies oxygenated air. These may be classified as your high-end ventilators.
-
Bag ventilator is a volume-controlled device where the CPAP and BI-PAP are pressure controlled. The Bag ventilator's oxygenated air output is only on during inhalation and not exhalation. This type of ventilation is classified as an assistive breathing equipment and may be designated as your low-end ventilator.
A bag valve mask (BVM), sometimes known by the proprietary name Ambu bag or generically as a manual resuscitator or "self-inflating bag", is a hand-held device commonly used to provide positive pressure ventilation to patients who are not breathing or not breathing adequately as seen below. The device is a required part of resuscitation kits for trained professionals in out-of-hospital settings (such as ambulance crews) and is also frequently used in hospitals as part of standard equipment found in emergency rooms or other critical care settings.

The BVM consists of a flexible air chamber (the "bag"), attached to a face mask via a shutter valve. When the face mask is properly applied and the "bag" is manually squeezed, the device forces air through into the patient's lungs; when the bag is released, it self-inflates from its other end, drawing in either ambient air or a low-pressure oxygen flow supplied by a regulated cylinder, while also allowing the patient's lungs to deflate to the ambient environment (not the bag) past the one-way valve.
Looking into the technical aspects of a ventilator, there are three important parameters that must be adjusted in real time by the ventilator operator according to the condition of a patient. These parameters are the tidal volume, which is the air delivered to a patient, Breaths per minute (BPM) which is the rate of breaths in a minute and the inhalation/exhalation ratio (IE) which is the time delay between an inhalation cycle and an exhalation cycle.
The parameters of the ventilator that will be produced will be for an adult patient and thus the key technical specification for an adult patient on a ventilator are a tidal volume ranging from 200mL to 800mL, 6 to 40 breaths per minute and an IE ratio ranging from 1:1 to 1:4 with intervals of 1.
Livingstone Hospital Ventilator Training
In the beginning of 2021, in-depth research was conducted on ventilators to ensure that the operational procedure and the characteristic of ventilators were understood extensively before the project can proceed.
The whole AEDG team took part in a theory and hands-on training day at a local government hospital Livingstone Hospital where the team were guests of the Head of ICU, Dr Liz van der Merwe, and her incredible staff.
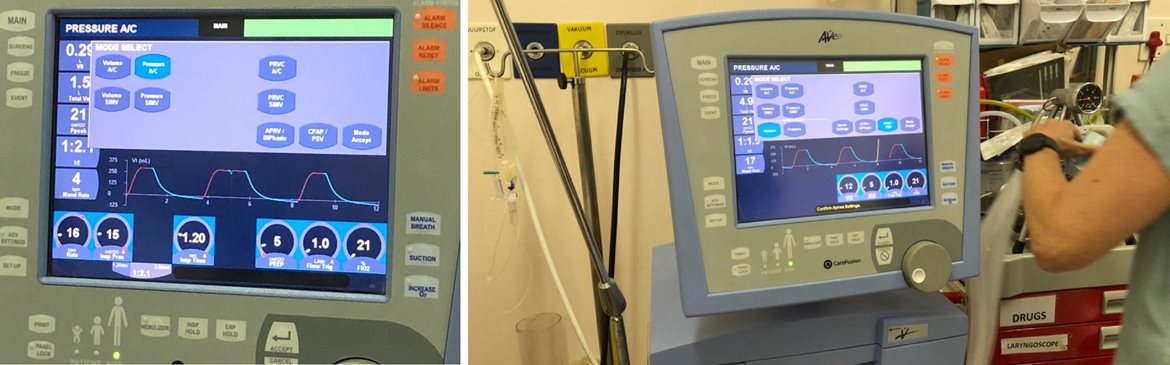
a typical expensive high-end full BiPAP multifunctional Ventilator as used on crititcal cases in an ICU
The morning training session featured an overview of Ventilator and Respiratory basics, which was invaluable as an insight as to what is required in the real-world, and the afternoon session featured full exposure to the operational procedure for various ventilators and related accessories, from the most basic to the most sophisticated.
This was followed by a tour through the ICU wards, showing the use of the various equipment in-situ with some severely afflicted patients, whilst also detailing the day-to-day challenges that faced HCW in the midst of a global pandemic. It was a very sobering and valuable experience for all the team as the hard realization of what these doctors face every minute of the day came home.

the visit to the Livingstone Hospital ICU was a harrowing but rewarding experience
Standards & Acceptable Applications
The design of the ventilator must adhere to the certain ISO standards for it to be deemed satisfactory for the usage on patients. In addition, in South Africa, any medical device must gain certification through a standardization body known as SAPRA.
Standards the ventilator should adhere to are as follows:
-
ISO 13485 which is the standard for manufacture of medical devices and good manufacturing practice.
-
ISO 18562 which is the biocompatibility evaluation of breathing gas pathways in healthcare applications.
-
ISO 20789 which is the standard for respiratory equipment.
-
ISO 17510 which is the standard for sleep apnea breathing therapy including masks and application accessories.
-
IEC 60601-1-1 which is a general safety standard for medical electrical equipment.
-
IEC 60601-1-2 which is general standard for requirement of basic safety and essential performance of medical electrical equipment such as electromagnetic capability.
In addition to the standards above the following requirements will be needed:
-
external surfaces of the ventilator must be cleanable using approved disinfectants.
-
must have a battery backup in case of power outage that can last a time greater than 20 mins.
-
all parts of the breathing system that meet patients must be single use.
-
materials used in all components of breathing system in gas pathway must be evaluated to ISO 18562-1
-
Ventilator and parts must have adequate mechanical strength when subjected to a mechanical stress caused by normal use.
-
Ventilator accuracy must be verified.
-
pressure relief must be verified.
-
closed sectioning test must be completed.
-
must have clear labelling which are permanent in accordance with ISO 7010.
-
14-day duty test cycle must be completed at different loading conditions.
Furthermore, an intensive amount of research has been conducted on Additive Manufacturing (AM) in aspects specifically on the process that will be used to manufacture the ventilators. Print materials may vary from metals (various alloys), plastics, thermoplastics, ceramics, biochemicals and even epoxies.
AM was utilized in this project as mode for prototyping as it presented an affordable option to quickly generate different prototypes for testing. AM can fast-track the design process considerably compared to using conventional manufacturing modes such as machining. All parts were printed on our in-house state-of-the-art Markforged desktop AM machines.
Extensive research has also been conducted on materials that will be used in the manufacturing of the ventilators. The material used to additively manufacture parts of the ventilator, will be OnyxⓇ which is a composite of Nylon (PA6) and short, chopped Carbon fibres. OnyxⓇ is slightly more expensive than a conventional material such as Acrylonitrile Butadiene Styrene (ABS) which is a thermoplastic polymer, however it does give us a higher degree of strength with is a favourable property for this application.
Design, Fatigue & Dynamic Simulation
Fatigue hand calculations were performed in conjunction with the fatigue dynamic simulation. Continuous iterative dynamic simulations have been performed with adjusted input parameters to determine optimal geometric design parameters for a reasonable design life. In addition to the calculations performed early on the gearbox, calculations have been performed and updated regarding the forces acting on the Ambu-bag and the power required from the motor.
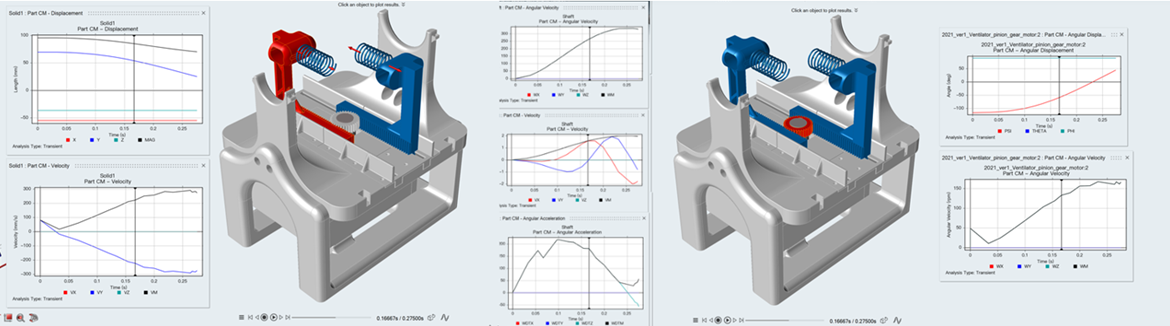
early dynamic analysis to characterize the capability of the pusher arms
Further hand calculations have been performed to determine the correct shaft diameters within the gearbox, the whole process of determining the resultant forces within the static loading scenario of the shaft within the gearbox has been conducted including shear force bending moment diagrams, and the complete modified Goodman shaft design process has been performed.
Altair Activate & Compose Training Sessions
The whole team in collaboration with Altair took up a course on Altair Activate and Compose software platforms, which enabled us to perform in depth dynamic simulations on specific parts of the ventilator such as the pusher arms and the Ambu-bag. By learning this software, ideally, we will be able to run a nonlinear dynamic simulation of the pusher arms and the Ambu-bag to determine the pressure and forces acting on the Ambu-bag and Pusher arms due to compression.
We can then determine the deformability of the air bag and the decrease in volume of the bag. This will enable a benchmark simulation from which future simulation data can be based to see if any improvement in performance has been achieved.
Following that, an optimization and dynamic stress analysis simulation can then be employed based on the following:
1. Pusher arm pressure plates that are in contact with the Ambu-bag.
a. by optimizing the shape, reduction of the back pressure on the pusher arms as well as the forces acting on the pusher arms can be iterated.
2. Run an optimization of the sliding contact the pusher arms move in, to reduce the friction thus allowing the pusher arms to move much smoother.
3. Run an analysis of the meshing system of the spur gear and rack on the pusher arm to determine excessive noise in the system.
Once the above simulations have been completed, the initial simulations could be re-iterated to check whether there has been any improvement from the original vanilla prototype.
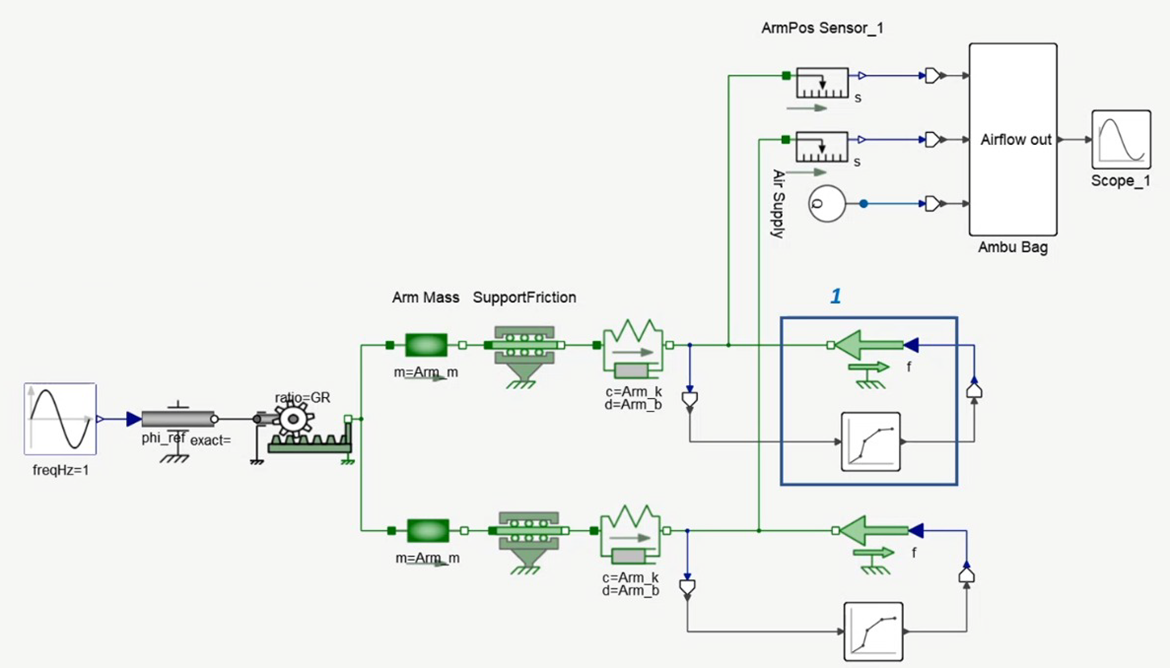
systems modelling as utilized in Altair Activate
Hardware Acquisition
An HMI interface was ordered that will help integrate all the electronics within the ventilator with an in-built touchscreen interface allowing easier adjustment of system variables.
The initiation of the complete 3D printing of two prototypes that are fully operational was also instituted; this allowed the team to get a more hands-on approach to the physical ventilator device and allowed the team to visibly identify possible improvement to the device away from a pure computational platform.
Sensors were ordered, including pressure sensors and flow sensors. These sensors allowed the ventilator to advance from a CPAP machine to a functional BiPAP machine. Furthermore, these sensors can be used to perform physical tests on the ventilator from which data can be extracted and compared to simulation results

the HMI interface in its raw state prior to programming
Coding & Electronics
Following the decision to recode the Ventilator from scratch, all the necessary information and variables were re-evaluated & established for the recoding of the base version ventilator. It was decided that the coding will primarily consists of the first three primary variables of tidal volume, BPM, and IE ratio.
Once the basic coding was complete, and the ventilator was performing as intended, more advanced coding will be performed to include the last variable of PEEP which will need external pressure sensors to be integrated into the ventilator for data extraction and analysis. Additional requirements such as the specs of the motor, specifications of the articulation system and the specifications on the ordered HMI were also ring-fenced at this stage to enable smooth development going forward.

(left) testing the basic functionality of the controller, and (right) the assembly of the microcontroller itself
The basic functionality of the ventilator was tested, and the coding was uploaded to the microcontroller controlling the articulation system of the ventilator. The basic movement of inflation and deflation of the Ambu bag was achieved.
Currently, further development on the HMI is occurring which can then be used to integrate the whole system together ensuring that the ventilator has adjustable variables through a touch screen display.
Printing & Assembly of the Base Version
The base version of the ventilator was printed and assembled featuring all updates and improvements - all parts were printed on the MF printers in Onyx material. The assembly of the ventilator included the press-fitting of brass M3 inserts which allowed components to be screwed on and rigidly kept in place. The servo motor was also integrated onto the printed components which is the main driver for the articulation system.

the updated Am printed base
Additional ventilator accessories have been purchased such as Ambu-Bags, breathing circuits and a test lung which will be very useful during the testing phase. Servo motors have also been ordered for integration into the articulation system as well as hardened brass inserts and fasteners for the assembly and fastening of components, ensuring required tolerances and fits.

(left) servo motor integrated into base, and (right) the assembled base
HMI Interface
The coding of the vanilla prototype was completed, and we had a fully functional CPAP Ventilator machine.
A touchscreen HMI was acquired and was configured to control the three main variables of the ventilator which are: Tidal volume, IE ratio and BPM.
The HMI was also reprogrammed within the coding of the Ventilator microcontroller to ensure the communication protocols between the two were ironclad - this was particularly challenging as the HMI used a conflicting protocol.

The touchscreen HMI Interface
There were many challenges that were faced here which took some time to debug, one of the issues included was that the readings from the HMI were not being recognized by the ventilator microcontroller but eventually through some coding conversions we were able to achieve communication between the two devices.
Furthermore, a circuit was designed and built which integrated all the necessary hardware such as the HMI screen, the power supply, the microcontroller, and few safety devices.
Design Updates on Vanilla Prototype
The Ventilators base was redesigned to cover all the internal electronics making the machine look more professional and presentable. The new base also enables more electronics to be placed without having to redesign for more space.
Pressure equipment was ordered such as the PEEP valve which monitors the back pressure within the system. Tubing, HEPA filters, and masks were also purchased.
A test lung was also acquired to help with the testing of the ventilator. Addition of these additional pressure hardware took the ventilator to midway between a CPAP machine and a BIPAP machine.
To convert this machine into a fully functional BIPAP machine, a few more sensors were then integrated, and the coding has been modified to include pressure monitoring, pressure triggering, pressure alarms and assistive breathing.
Most of the modification moving forward was then done on the firmware of the device in order to integrate these new components.

the upgraded iteration of the Ventilator heading to a functional BiPAP version
Control Aspects
The Final stage envisioned for this project was then completed, which was to develop a CPAP/Bi-BAP ventilator that met the clinical requirements for low-cost mechanical ventilation.
Once the CPAP mode of ventilation was achieved, BIPAP ventilation was achieved through the addition of pressure sensors and modification of firmware. The firmware was updated to include assistive breathing which enabled the ventilator to operate in sync with the patients breathing pattern.
The pressure sensors enabled the ventilator to operate on two different pressure gradients, a higher pressure on inhalation and a lower pressure of exhalation.
CPAP and BiPAP modes are achieved & integrated onto the HMI
The coding for the full-blown Bi-PAP ventilator was next completed including the modification of the HMI display to enable the operation of the ventilator between the CPAP mode and Bi-BPAP mode. Furthermore, within the Bi-PAP mode the HMI displayed data that was extracted from various sensors within the system to allow easy monitoring of patients.
Calibration of the sensors within the system was a major challenge, however, through the purchasing of a digital pressure manometer, the calibration of the sensors through modification of firmware was achieved.
Through the variety of electronic challenges faced by the student team, it was clear that a lot had been learnt by trial and error iro the serious need for balance between electronics and mechanical design within students, as the global trend towards the integration of electronics, sensors, IOT and software within machines has become mainstream.
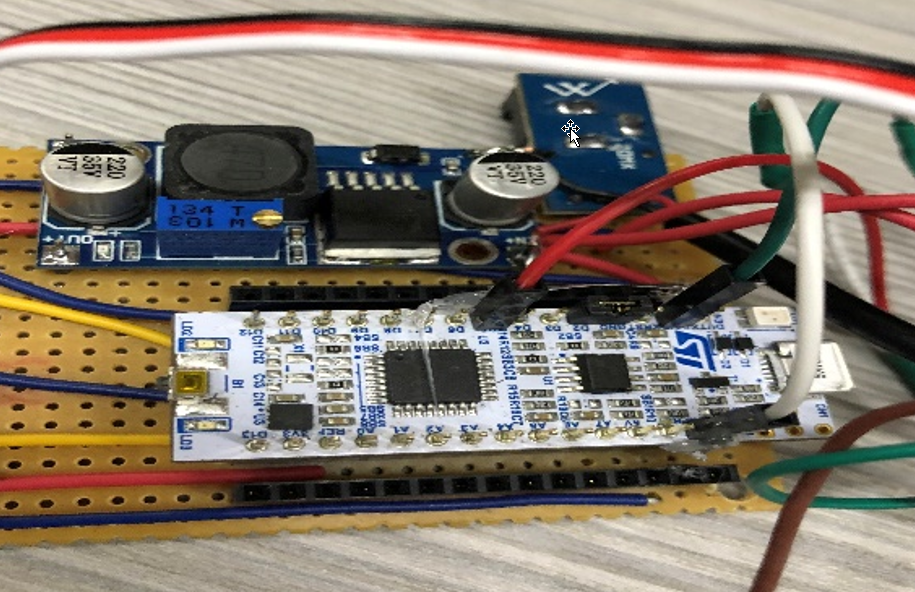
completed electronics being tested successfully
The BiPAP Ventilator was now complete iro the Project Objectives and was next prepared for demonstration at the upcoming RAPDASA Conference.
RAPDASA Conference
The BiPAP Ventilator was presented as a paper and presentation at the 2021 RAPDASA Conference at the CSIR ICC in Pretoria. The AEDG team was hosted at the EOS/Rapid3D stand throughout the event - much appreciation to all at Rapid3D...
The RAPDASA Conference is the premier Rapid Prototyping Development Conference in the Southern Hemisphere and is held annually to bring together the very latest in AM-related technological development services, hardware, software, and personnel from across the globe right here on South African soil. The three-day event consisted of many presentations and displays showcasing the state-of-the-art in the AM game.
The RAPDASA Conference and presentations has exposed the students to different ideas and has initiated collaboration among different disciples. It has also benefited students by allowing them to make connections with people from all over the world which is a huge benefit moving into industry.

(left) team members with our display table showing the Ventilator front and centre, (right) Zaahid busy with the presentation of his work
Moving Forward
Following the formal conclusion of the BiPAP Ventilator project iro its initial objectives, Zaahid has also registered for his Masters and will now continue to use the BiPAP Ventilator as a test bench for further optimization of the Pusher Arms in respect of trying to further develop and optimize the efficiency of the device - this will be a continuous project through 2022 due for completion by the end of the year.
This will ensure that the Ventilator Project has life beyond its projected funding span, and may open up other opportunites going forward.
Contributers
Huge thanks & appreciation must go out to all these individuals for their completely selfless contribution to this, be it for information, advice, their time, their contacts, their services, or whatever contribution they made, no matter how big or small.
Custom Works (Arno Seyfert)
Rapid3D (Dave & Pauline Bullock: Co-Founders & Directors, Lynton Dent: Engineer)
NMU Mech Eng (Dr William Rall: Mechanical Design)
AEDG Team (Clive Hands, Jode Fourie, Muhammed Lookmanjee, Daniel Trask, Wian van Aswegen, Zaahid Imran)
Livingstone Hospital (Dr Liz van der Merwe: Head ICU, Dr Bryan Theunissen: Orthopaedic Surgeon)
Altair (Nic Minnaar: Engineering Analyst)
Voltera (Katarina Illic: Co-Founder & Director)
Stopforth Robotics (Prof Riaan Stopforth: UKZN)
Charl Rossouw (Programming & Electronics Advisor)
Software Utilized:
SolidWorks (Design & CFD)
Inventor (Design)
SimSolid (FEA)
Altair Inspire (topology optimisation)
Altair Activate (Systems Simulation)
Altair Inspire Motion (dynamic simlation)
Altair Inspire Studio (modelling & visualization)
Voltera V-One (PCB printer platform)
Eiger (AM Printer Slicing platform)
Visual Studio Code (coding)
Arduino IDE (Arduino programming)
Nextions IDE (microcontroller programming)
easyEDA (PCB design)