
Objectives
A meeting was held in February 2022 at Livingstone Hospital with the Anaesthesiology staff team members, Dr Lorenzo Boretti and Dr Marné Page, to discuss potential small projects that the AEDG student team could collaborate on going forward...projects that were achievable in short-term time periods, that could be conceptualised, designed, manufactured, tested and implemented over weeks....and which could make a difference to day-to-day challenges experienced in the hospital.
Several interesting and achievable projects came to light, one of which was the eventual Blood Alert device detailed here.
The initial idea for the Blood Alert device was to autonomously monitor blood levels in a collection vessel under various conditions, and then to alert medical staff once the blood levels had reached various predetermined levels so that action could be taken to help save lives.
The purpose of the Blood Alert device is related to Patient Blood Management (PBM) where the move is to optimize the patient's own blood and prevent the need for transfusion of donated blood. The Blood Alert system is an early-detection or awareness device that contributes to the awareness of blood loss before it becomes significant.
In so doing the responsible clinicians can intervene earlier and prevent further blood loss and subsequent transfusion of donated blood.
Concept & Development
A follow-up meeting was held with Dr Page to get eyes on the actual equipment used specifically in procedures for haemorrhage control, as this where the device would be utilised. The team was able to measure and photograph the various collection devices used for monitoring the severity of blood loss.

the various collection devices used in haemorrhage fluid collection in situ in the hospitals
The initial concepts were to create a conformal band that could easily slip onto the collection vessels and could be fastened into place at predetermined positions depending on individual requirements. Further, that embedded sensors inside the device could then detect the blood level and emit a warning alarm.
Various configurations were sketched out with the design objectives of a concept that...
-
...had minimal footprint,
-
...would fit onto the vessels as inobtrusively as possibly,
-
...would not interfere with the functioning of the vessels,
-
...could still be operated by staff with surgical gloves on...
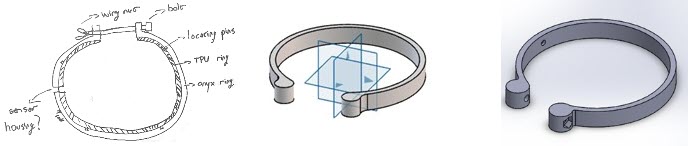
initial concepts & base model
This concept was the basis for the Blood Alert device going forward as further design details were developed.
Electronics Design
Initially an Arduino-controlled option was considered, but it soon became apparent that is was not necessary to have all the bells and whistles that the Arduino presented, nor a microcontroller capability. Furthermore, the Arduino presented quite a large footprint and the Design Team soon realised that we could achieve what we wanted with a custom passive PCB design, and so the design very quickly gravitated towards this type of solution.
After some research online for a similar type of PCB functionality, testing was started with our in-house electronics resources, and very soon a makeshift configuration was set up on a breadboard and tested with one of the supplied collection vessels from the hospital. This featured an adjustable photoresistor triggering an alarm when a change of collector contents was detected; this would then alert the medical staff that their threshold level had been reached.
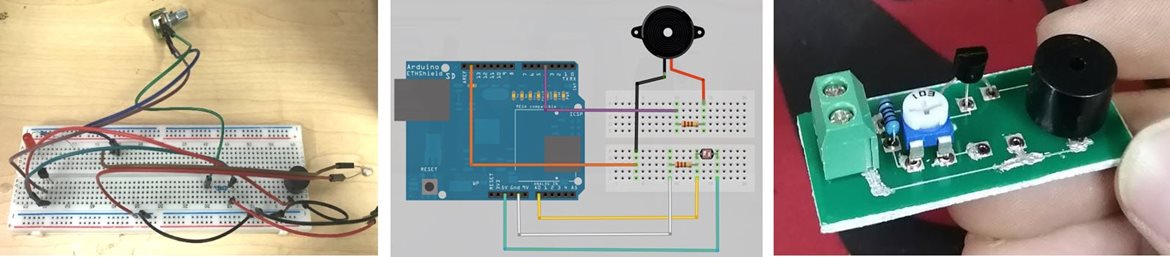
initial circuit layout and test PCB
This was then further honed down and simplified into a PCB design on the EasyEDA platform, and an initial rough test PCB was manufactured as a testbench for verification of the functionality. After further testing and optimisation of the circuit, attention was given to reducing the PCB footprint so that it would occupy minimum space on the final structure and be as compact as possible.
Once this was achieved to the team's satisfaction, the final PCB design was commited to printing on the Voltera V-One, and then tested further as a completed unit. The video below depicts the printing/manufacture process of the finalised PCB on the Voltera.
printing the PCB on the Voltera V-One printer
The final PCB was then cut to size for final insertion into the PCB housing and connection to the interior wiring connecting the battery and the ON/OFF switch.
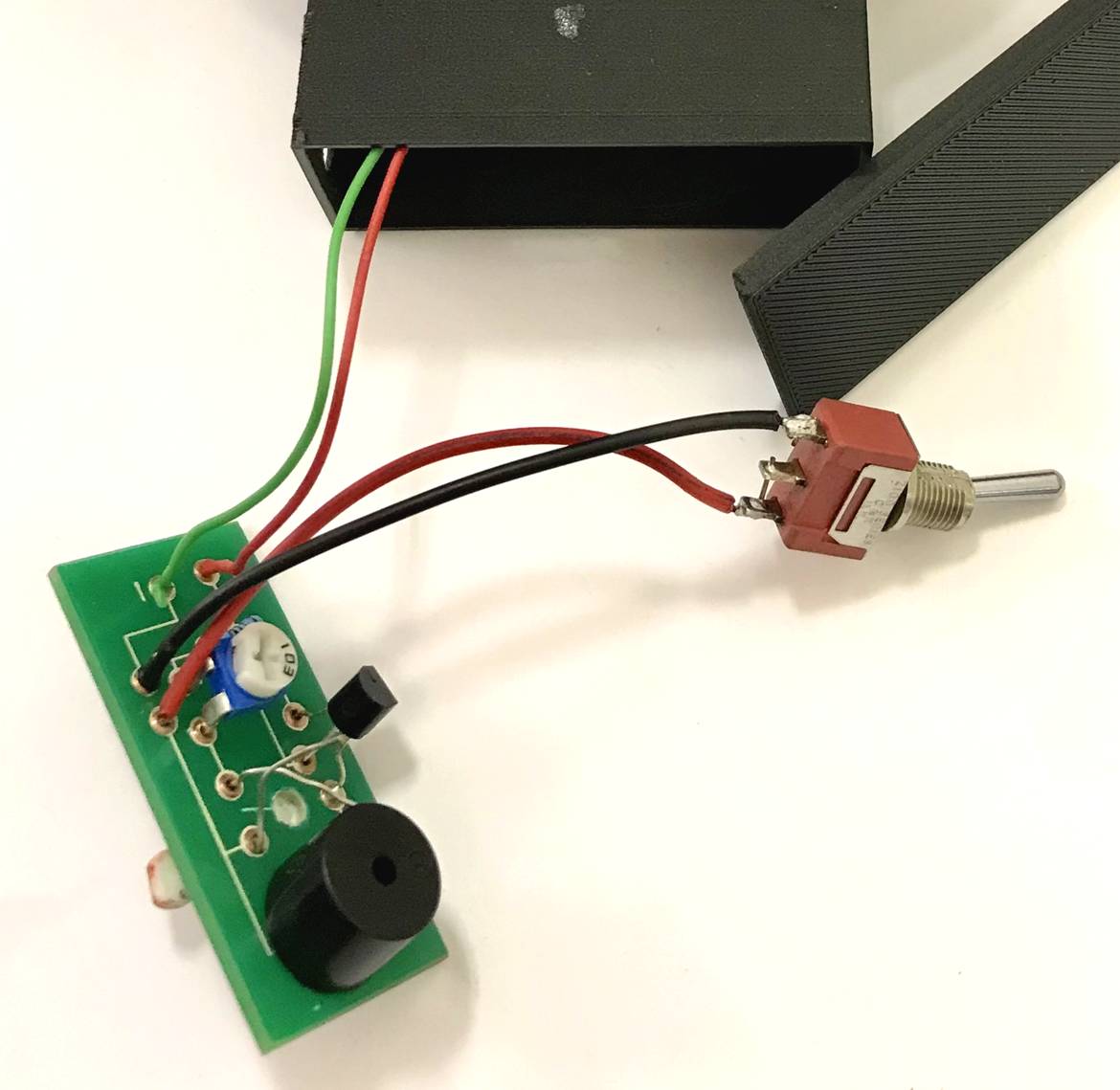
the final PCB with all connected components ready for integration into the device
Structural Design
Design Objectives
It was decided that the design would feature the following criteria:
-
an adjustable band to allow for various manual vertical positions on the collection vessel
-
this would be achieved through a simple adjustable threaded rod with a wingnut attachment
-
a PCB enclosure shrinkwrapped down to a minimal volume around the PCB
-
internal conformal channels to house the wiring for the electronics
-
a coin battery enclosure to allow for simple replacement of the battery
-
an ON/OFF switch that would be easily operable by someone using surgical gloves
-
the entire structure would have to encompass Design for Additive Manufacturing (DfAM) principles enabling it to be 3D printed using current in-house technology, minimising or eliminating any support structures
-
minimal separate components simplifying the assembly
-
rugged and simple to prevent potential damage and to minimise maintenance
The design features are displayed in the semi-transparent model render below...all design objectives were able to be met with this design.
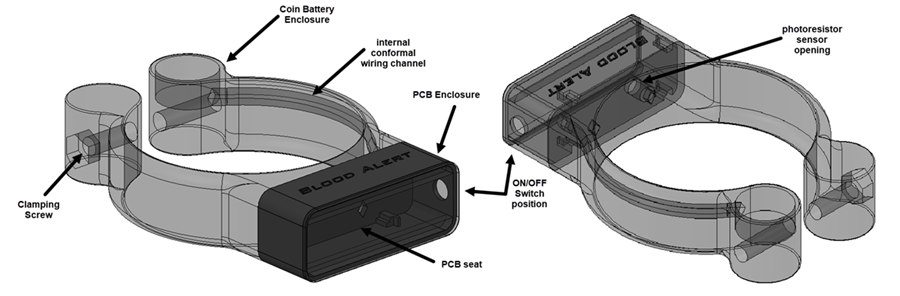
internal details of the Blood Alert device showing conformal channels for cabling
Function
The device works using a photoresistor embedded inside the clamping band. The photoresistor is connected to a potentiometer which is used to control the activation sensitivity of the rest of the circuit. The rest of the circuit consists of a transistor, a passive buzzer, a resistor and a battery that powers the entire device.
When light to the photoresistor gets blocked off by the blood inside the collection vessel, the rest of the circuit gets activated and the buzzer alerts the user that the blood has reached the pre-set level. The components are connected to each other on a small custom PCB board housed inside the device. The device is activated using a simple toggle switch on the side of the PCB enclosure.
The adjustable ring can fit on any suitable cylinder by adjusting the diameter using the bolt and wing nut. To secure the device, the user simply tightens the wing nut.
Prototype Testing & Design Iteration
Testing
The initial prototype worked as anticipated, but needed to be optimised to work on the collection vessels with real blood. The device was initially too sensitive, so the potentiometer on the PCB needed to be adjusted so that the alarm would only go off once the photoresistor was blocked off by the blood instead of triggering the alarm for any light changes on the photoresistor.
To replicate blood, a dark coffee mixture was used to simulate the blood flowing into the vessel. The Blood Alert device was clamped onto one of the test vessels and a makeshift vacuum supply (ie. siphoning coffee through a tube) was created to simulate blood accumulating in the collection vessel.
The vessel was filled up with coffee to the level where the Blood Alert was positioned. The resistance was then adjusted so that the buzzer was only triggered when the dark liquid inside the vessel reached the appropriate level.
testing the device with 'blood coffee'
Design Iteration
Different potentiometers were tested to figure out which one would give the optimal voltage to activate the buzzer by the fluid in the collection vessel; three different level potentiometers were tested, before deciding which worked the best and had the best sensitivity for the intended purpose.
The other components (buzzer, resistor, transistor, photoresistor) worked well and did not need to be changed. The initial prototype featured a straight circular hole to house the bolt that tightens the device to the blood collection vessels. This caused the bolt to pinch when the device was at the maximum diameter of the blood collection vessel. An elliptical slot was introduced to allow some play when needed.
It was also found that the Onyx material used for the enclosure overdamped the sound from the buzzer, which was not ideal seeing that the buzzer needed to be loud enough to alert the doctors that the blood had reached the threshold limit. A speaker grill was included in the updated PCB enclosure cap to allow the sound to propagate better.
An LED was also introduced to indicate if the device was on or off to prevent unnecessary use of the battery.
The initial prototype did not have branding or ON/OFF indications, so this was included in the second iteration. Battery location and voltage indications were also included on the battery cap to ensure that the correct battery was used.
An initial challenge was that it was difficult to remove the press-fit battery holder cap, so a small indent was created to make it easier to remove the battery cap when the battery needed replacing. All these changes were made to both versions of the device, one for the smaller collection vessel and one for the bigger collection vessel.
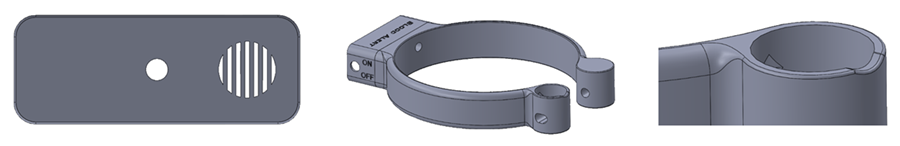
(left to right): the updated PCB enclosure cover, the embossed branding on the enclosure, and the battery cap indent
Future work/Improvements
Some future improvements that will be made to the next design iteration include:
-
a better battery connector (because the spring mechanism might become unreliable or damage the battery), and
-
a redesign of the battery holder cap from a press-fit to a twist lock cap (to better secure the battery).
Prototype Handover
The completed initial prototypes were handed over to Dr Lorenzo Boretti at Livingstone Hospital on Thursday 21st April 2022 - one for each size of collection vessel, with both featuring exactly the same functionality.
These will now undergo further testing on-site to gauge where further modifications and updates are needed to finalise the device...

(l-r): Jode Fourie, Dr Lorenzo Boretti, & Brian Jack hand over the two initial prototypes for testing
Feedback & Further Development
The Blood Alert devices are now in on-site testing to gauge their effectiveness and to also gauge where further updates in the design are necessary.
Once the feedback has been received from the medical staff, further refinements will be made to the device and this section wil then be further updated over time.
Final Product
This section will be updated once the on-site testing at Livingstone Hospital has occured and feedback from the medical staff has been further acted upon.
The plan thereafter is to make the technology available to hospitals wherever it may be needed...
Media
2022-04-27 article from the Herald:

Contributers
Huge thanks & appreciation must go out to all these individuals for their contribution to the project, be it for information, advice, their time, their contacts, their services, or whatever contribution they made, no matter how big or small.
AEDG Design Engineers (Jode Fourie & Brian Jack)
Livingstone Hospital (Dr Lorenzo Boretti & Dr Marné Page)
Hardware Utilized:
Markforged Onyx One (3D Printing)
MarkForged Mark Two (3D Printing)
Voltera V-One (PCB Manufacture)
Software Utilized:
SolidWorks (
Modelling)
3DExperience (Concept Design)
Altair Inspire Studio (modelling & visualization)
Voltera (PCB-Printer PCB manufacture)
Eiger (MarkForged AM Printer Slicing platform)
easyEDA (PCB design)